The wavelength is given by the equation wavelength (nm) = h*c/E where h*c is 1.23984 KeV nm and E is the photon energy in KeV. The lightest element of interest , Mg, has an fluorescence x-ray energy of 1.253 KeV which would have a wavelength of .798 nm. We would not be able to excite fluorescence above the source energy which is 59.7 KeV or .0208 nm. If you wish to see carbon, its emission is .277KeV or 4.476nm. I expect that the carbon will be fairly transparent to the source photons though.
Thanks RJ - it gets us closer.
I got h * c = 1.9864458121E-25 J.m , which is exactly the value found in Wikipedia "Planck Constant"
That Planck constant h = 6.626070E-34 is an exact value, defined, the basis for fixing the kilogram.
To use your value for h * c, and divide by c
1.23984/c = 4.1356610779e-09 which is the number I see in the Wiki.
The given usual value is 4.135667696E-15 eV.s, but that's OK. there were 5 decimals to start with.
The value is also exact, but not expressible as a finite decimal, so approximated to 9 decimal place
The e-9 instead of e-15 is because we go direct to nanometres, and use KeV to begin with.
I get it that the value you used has the eV = 1.602176634E-19 built in.
- - - - - - - - - - -
Looking at some of the solid state sensors suggested by
@homebrewed is interesting. Unfortunately, the areas are really small compared to common PMT tubes. Generally multiples of 6mm square.
MICROFC-60035-SMT-TR1 6mm x 6mm, tileable $72,92
MICROFJ-30035-TSV-TR 3mm x 3mm tileable $ 23.59
.. and so on
The selection is here -->
Mouser.com ON Semiconductor Photodiodes
There is also a range of TO3 size windowed package avalanche photodiodes. Generally expensive .
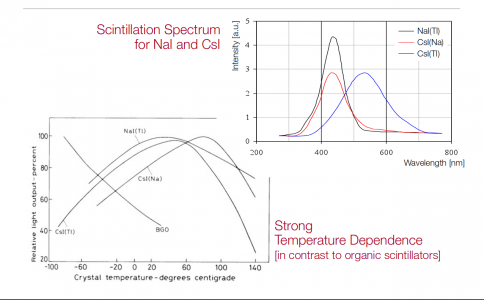
The other things I have learned about scintillators is CsI is much more rugged, and a good bit less hygroscopic than NaI. The sensitivity of CsI is much lower. PMT tubes need magnetic shielding, mu-metal as well as lead at the front. Finally, they have to use a holder that excludes all light when put over, or up against, the test sample.
en.wikipedia.org