- Joined
- May 27, 2016
- Messages
- 3,479
I have some chunks of nice steel, 31mm thick and 120mm x 60mm (about 1.2" thick and 4.7" x 2.3").
The 31mm apart faces are parallel to within a tenth thou, so they are sometimes handy as a sort of fat parallel, but the perimeters are not as in a rectangular block. They have the profile of whatever hydraulic pump kit they were originally destined for.
They don't rust - so not just some nearly tool steel.
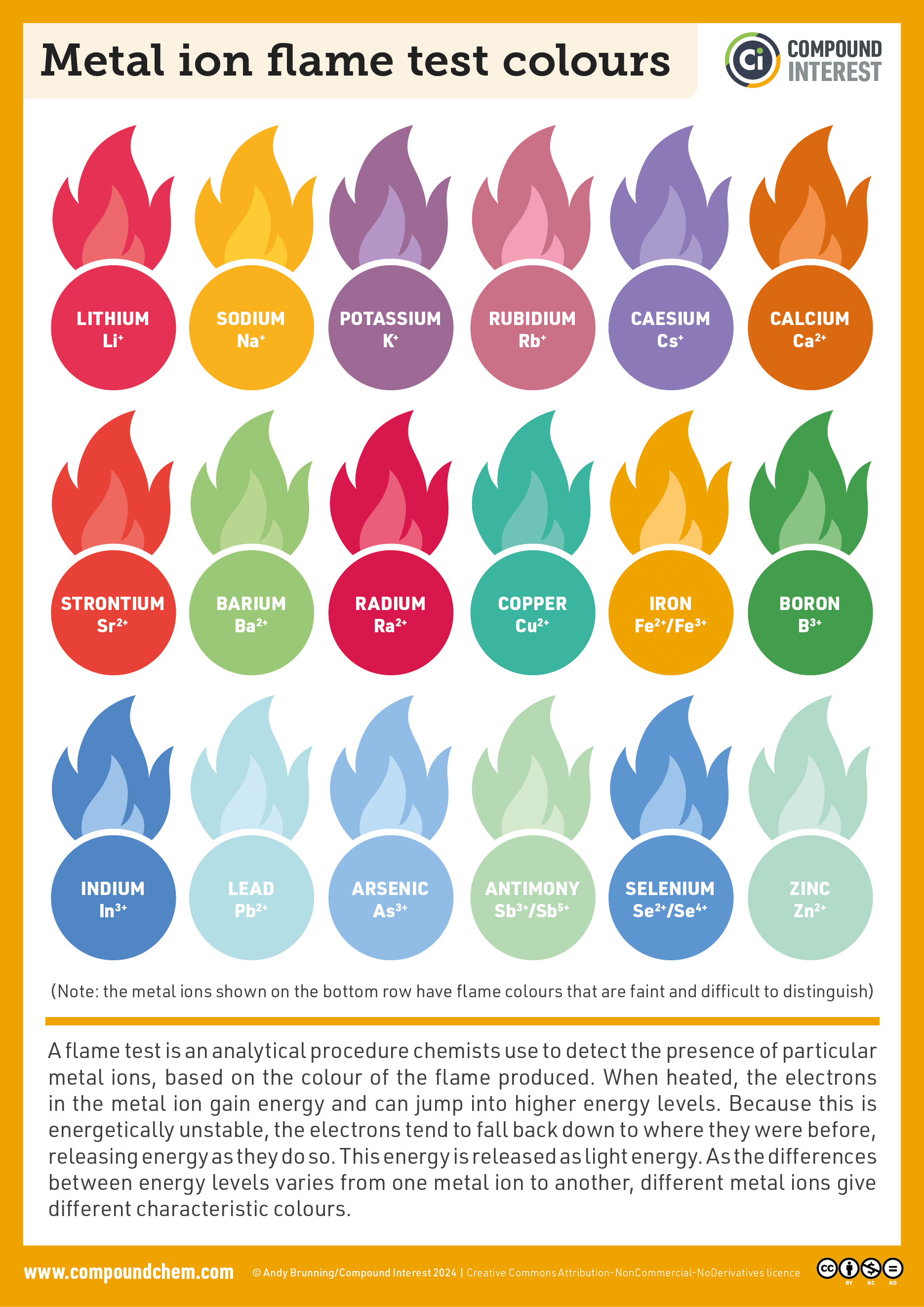
They are magnetic, so mostly iron in there.
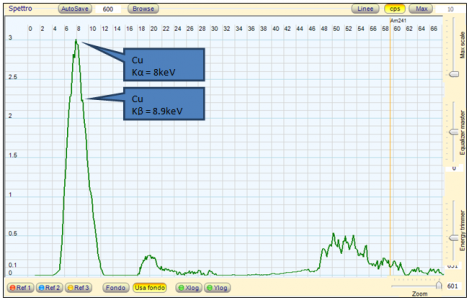
It seems there is not much between the "spark test" on a grinder, and some expensive kind of analysis.
I can't afford one of those FLIR things which are only cost-effective if they are identifying exotic stuff all all day long.
Trying for density once let me identify a nice chunk of titanium, but I did have some other clues.
Is there some lower cost way of figuring out what is in the alloy?
The 31mm apart faces are parallel to within a tenth thou, so they are sometimes handy as a sort of fat parallel, but the perimeters are not as in a rectangular block. They have the profile of whatever hydraulic pump kit they were originally destined for.
They don't rust - so not just some nearly tool steel.
They are magnetic, so mostly iron in there.
It seems there is not much between the "spark test" on a grinder, and some expensive kind of analysis.
I can't afford one of those FLIR things which are only cost-effective if they are identifying exotic stuff all all day long.
Trying for density once let me identify a nice chunk of titanium, but I did have some other clues.
Is there some lower cost way of figuring out what is in the alloy?