More on ball-point pen inks.
Yes - I am still riding this experiment, in the sense of 1st try been standing around for a few days, left to itself, because I have not got around to clearing it up, and I noted changes. My wife thinks I'm in need of help..
"I don't know what's got into him"?
"Why on Earth would he want to be scratching lines and writing on metal,and then keep wiping it off"?
"He does it all reversed! He gets the ink out of the pens, and spreads it out, then he writes in it with something sharp"!
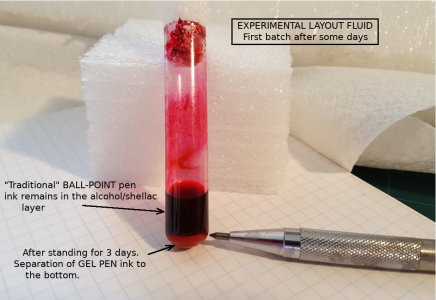
Gel Pen ink does not work- but ballpoint ink does!
At least - not in the shellac/alcohol mix. It appears to at first, but the painted layer has a grainy top surface, and a thick rim boundary. If left for long enough, gravity separates it. This would have been seen sooner if I had a test-tube centrifuge. [Is that maybe a machinist aspirational project?].
View attachment 361316 _
View attachment 361317
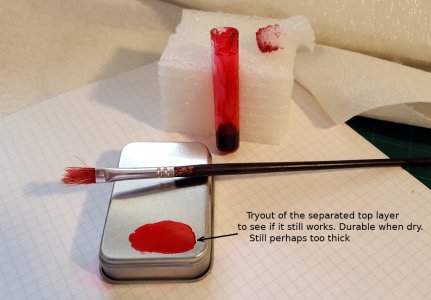
I did try to paint some metal with some of the separated top layer, which I reasoned would contain the traditional ball-point ink. It still appears to work in getting a quick drying layer, but this is not a good test. I should have dumped that batch long ago, and properly checked out a, clean fluid mix.
It is not yet quite right. I think the layer might still be too thick, and on that plasticized tin-plate lid from a spice can, lines would scribe just fine with a scalpel. With a carbide scriber, the layer hangs onto itself better than to the metal, so drags a line that tears a little "wider" than wanted.
I think the layer is perhaps too thick, and needs more alcohol.
The coverage is completely enough. I also painted up a layer about 2" x 1", and hardly used more than a couple of drops out of that top layer, which all started with half a gram of shellac. When dry, it's durable, and does not come off onto the fingers or clothes, When it wipes off with alcohol, it comes off easy, except for a resisting faint fine line around the paint boundary, which seems to need some more hard rubbing.
Other fluids
One suggested by
@homebrewed is to get some expanded polystyrene foam (parcel packing, wall insulation, lightweight feeble white stuff), and dissolve in acetone. I consider acetone to be harsher stuff than alcohol, and I wheeze up on the fumes, so I use a carbon filter mask, but anyway, the polystyrene magically disappears into it, and the stuff that is left does leave a layer, but the fluid evaporates and thickens by itself real fast. MEK evaporates slowly, but I am not going to use it.
So another experiment is mooted, using polystyrene packing